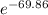Chemistry, 19.11.2019 02:31 valoiserika1229
For the reaction of oxygen and nitrogen to form nitric oxide, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh⁰rxn 180.5kj/mol δs⁰rxn 24.80j/(mol⋅k) a. calculate the temperature in kelvins above which this reaction is spontaneous b. the thermodynamic values from part a will be useful as you work through part b: δh⁰rxn 180.5kj/mol δs⁰rxn 24.80j/(mol⋅k) calculate the equilibrium constant for the following reaction at room temperature, 25 ∘c : n2(g)+o2(g)→2no(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 23.06.2019 15:30
Light travels through space at 186,282 miles per second and it takes about 1.3 seconds for light to travel from the moon to earth. which of the following is the correct method of finding the distance, in miles, between the moon and earth? add 186,282 and 1.3 divide 186,282 by 1.3 multiply 186,282 by 1.3 subtract 1.3 from 186,282
Answers: 1
You know the right answer?
For the reaction of oxygen and nitrogen to form nitric oxide, consider the following thermodynamic d...
Questions

History, 06.10.2020 06:01



Mathematics, 06.10.2020 06:01


Physics, 06.10.2020 06:01

Mathematics, 06.10.2020 06:01

Mathematics, 06.10.2020 06:01


Mathematics, 06.10.2020 06:01


History, 06.10.2020 06:01

English, 06.10.2020 06:01


Mathematics, 06.10.2020 06:01



Chemistry, 06.10.2020 06:01

Social Studies, 06.10.2020 06:01