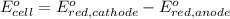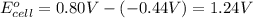Chemistry, 19.11.2019 01:31 burnsmykala23
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given that the standard reduction potential of ag+ to ag (s) is +0.80 v and the standard reduction potential of fe2+ to fe (s) is −0.44 v, calculate the standard cell potential, e°cell.−1.24 v1.24 v2.04 v0.36 v

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3


Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given...
Questions

Biology, 02.10.2019 07:30



History, 02.10.2019 07:30


Mathematics, 02.10.2019 07:30




Mathematics, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30

English, 02.10.2019 07:30

Biology, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30



History, 02.10.2019 07:30


Mathematics, 02.10.2019 07:30

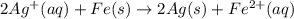
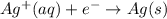
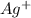 to Ag=
to Ag=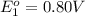
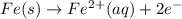
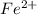 to Fe=
to Fe=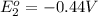
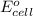 of the reaction, we use the equation:
of the reaction, we use the equation: