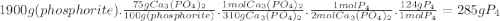Phosporus can be prepared from calcium phosphate by the following reaction: 2ca3(po4)2+6sio2+10c → 6casio3+p4+10co phosphorite is a mineral that contains ca3(po4)2 by mass? assume an excess of the other reactants plus other non-phosphorus-containing compounds. what is the maximum amount of p4 that can be produced from 1.9kg of phosphorite sample is 75% ca3(po4)2 by mass? assume an excess of the other reactants

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Phosporus can be prepared from calcium phosphate by the following reaction: 2ca3(po4)2+6sio2+10c →...
Questions

Mathematics, 29.07.2019 18:30


English, 29.07.2019 18:30



History, 29.07.2019 18:30


Mathematics, 29.07.2019 18:30

Mathematics, 29.07.2019 18:30

History, 29.07.2019 18:30

Mathematics, 29.07.2019 18:30


History, 29.07.2019 18:30

Health, 29.07.2019 18:30

History, 29.07.2019 18:30



Mathematics, 29.07.2019 18:30


Mathematics, 29.07.2019 18:30