
Nh4+ (aq) + no2- (aq) → n2 (g) + h2o (l) experiment [nh4+]i [no2-]i initial rate (m/s) 1 0.24 0.10 7.2 x 10-4 2 0.12 0.10 3.6 x 10-4 3 0.12 0.15 5.4 x 10-4 4 0.12 0.12 4.3 x 10-4 first determine the rate law and rate constant. under the same initial conditions as in experiment 4, calculate [nh4+] at 274 seconds after the start of the reaction. in this experiment, both reactants are present at the same initial concentration. the units should be m, and should be calculated to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Nh4+ (aq) + no2- (aq) → n2 (g) + h2o (l) experiment [nh4+]i [no2-]i initial rate (m/s) 1 0.24 0.10 7...
Questions

Mathematics, 05.08.2021 18:10

Mathematics, 05.08.2021 18:10


Mathematics, 05.08.2021 18:10



Chemistry, 05.08.2021 18:10

Mathematics, 05.08.2021 18:10

Mathematics, 05.08.2021 18:10

Mathematics, 05.08.2021 18:10



Mathematics, 05.08.2021 18:10

Mathematics, 05.08.2021 18:10

Mathematics, 05.08.2021 18:10





![\text{Rate}=k[NH_4^+][NO_2^-]](/tpl/images/0380/3184/ed258.png)
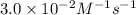
![[NH_4^+]](/tpl/images/0380/3184/5c46c.png) at 274 seconds after the start of the reaction is 0.0604 M
at 274 seconds after the start of the reaction is 0.0604 M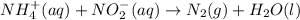
![\text{Rate}=k[NH_4^+]^a[NO_2^-]^b](/tpl/images/0380/3184/00132.png)
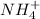
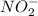
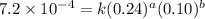 ....(1)
....(1)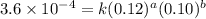 ....(2)
....(2)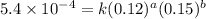 ....(3)
....(3)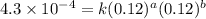 ....(4)
....(4)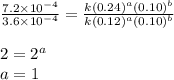
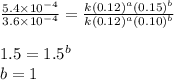
![\text{Rate}=k[NH_4^+]^1[NO_2^-]^1](/tpl/images/0380/3184/43151.png)
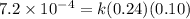
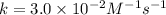
![[NO_2^-]](/tpl/images/0380/3184/10a69.png) are same and the reaction is 1st order for both.
are same and the reaction is 1st order for both.![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0380/3184/ccade.png)
![[A_t]](/tpl/images/0380/3184/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0380/3184/dc622.png) = initial concentration = 0.12 M
= initial concentration = 0.12 M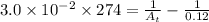
![[A_t]=0.0604M](/tpl/images/0380/3184/69b49.png)




