
Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochloric acid and trapping the gas produced in an inverted tube initially filled with water. if the total pressure of the gas in the collection tube is 729.7 mmhg at 25°c, what is the partial pressure of the hydrogen? the vapor pressure of water is 23.8 mmhg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochlor...
Questions

Biology, 24.08.2019 01:30


Chemistry, 24.08.2019 01:30






Engineering, 24.08.2019 01:30





Mathematics, 24.08.2019 01:30

Computers and Technology, 24.08.2019 01:30

Mathematics, 24.08.2019 01:30

English, 24.08.2019 01:30

Geography, 24.08.2019 01:30

History, 24.08.2019 01:30


 = 729.8 mmHg
= 729.8 mmHg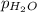 = 23.8 mmHg
= 23.8 mmHg


