
The reaction system: pocl3(g) pocl(g) + cl2(g) is at equilibrium. which of the following statements describes the behavior of the system if pocl is added to the container? (a) the forward reaction will proceed to establish equilibrium.(b) the reverse reaction will proceed to establish equilibrium.(c) the partial pressures of pocl3 and pocl will remain steady while the partial pressure of chlorine increases.(d) the partial pressure of chlorine remains steady while the partial pressures of pocl3 and pocl increase.(e) the partial pressure of chlorine will increase while the partial pressure of pocl decreases.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1



You know the right answer?
The reaction system: pocl3(g) pocl(g) + cl2(g) is at equilibrium. which of the following statements...
Questions


History, 11.12.2021 07:50



Mathematics, 11.12.2021 07:50



Chemistry, 11.12.2021 07:50



Health, 11.12.2021 07:50

History, 11.12.2021 07:50


Social Studies, 11.12.2021 07:50

Computers and Technology, 11.12.2021 07:50

Mathematics, 11.12.2021 07:50




History, 11.12.2021 08:00

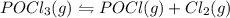
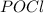 is added to the system
is added to the system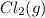 decreases and the partial pressure of
decreases and the partial pressure of 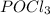 increases.
increases.

