Chemistry, 18.11.2019 23:31 senituliii
Aconcentration cell is one in which both the anode and cathode are the same but with different concentrations. calculate the cell potential with [zn2+] = 0.10 m for the cathode and the [zn2+] = 0.010 m for the anode?
a. + 0.06 v
b. - 0.03 v
c. + 0.03 v
d. 0.0 v

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Aconcentration cell is one in which both the anode and cathode are the same but with different conce...
Questions




History, 31.03.2021 18:10


Spanish, 31.03.2021 18:10



Physics, 31.03.2021 18:10



English, 31.03.2021 18:10







Mathematics, 31.03.2021 18:10

Health, 31.03.2021 18:10

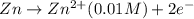
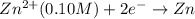
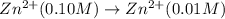
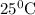 :
:![E_{cell}=\frac{-0.059}{n}log{\frac{[Zn^{2+}]_{0.01M}}{[Zn^{2+}]_{0.10M}}}](/tpl/images/0380/1872/f8c0a.png)