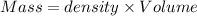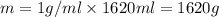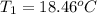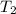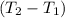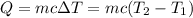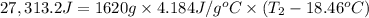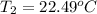Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of both solutions is the same at 18.46°c. the heat of neutralization when 1.00 mol of hno3 reacts with 0.500 mol ba(oh)2 is −56.2 kj/mol. assume that the densities and specific heats of the solution are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively). what is the final temperature of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a const...
Questions

Health, 21.09.2019 03:40

Physics, 21.09.2019 03:40

History, 21.09.2019 03:40

History, 21.09.2019 03:40


Mathematics, 21.09.2019 03:40

Mathematics, 21.09.2019 03:40


Health, 21.09.2019 03:40

Mathematics, 21.09.2019 03:40


English, 21.09.2019 03:50


Mathematics, 21.09.2019 03:50


History, 21.09.2019 03:50


Arts, 21.09.2019 03:50

Mathematics, 21.09.2019 03:50

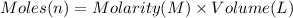
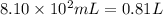
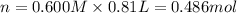
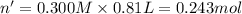
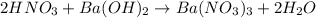
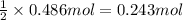 barium hydroxide.
barium hydroxide.