
Chemistry, 18.11.2019 21:31 angeline2004
How long of time in hours will it take to plate out 1.0 kg ni from aqueous ni2+ solution, if the electrical current is 102 a? where: ni2+(aq)+2e-→ni(s) view available hint(s) how long of time in hours will it take to plate out 1.0 kg ni from aqueous ni2+ solution, if the electrical current is 102 a? where: ni2+(aq)+2e-→ni(s) 90 hours 0.895 hours 8.95 hours 900 hours

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
How long of time in hours will it take to plate out 1.0 kg ni from aqueous ni2+ solution, if the ele...
Questions

Mathematics, 09.04.2021 19:00


Biology, 09.04.2021 19:00


English, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00

Biology, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00


Mathematics, 09.04.2021 19:00








Mathematics, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00

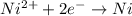
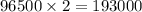 Coloumb of electricity deposits 1 mole of Nickel
Coloumb of electricity deposits 1 mole of Nickel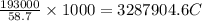 of electricity
of electricity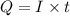
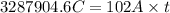
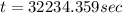
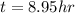 (1h=3600 sec)
(1h=3600 sec)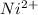 solution, if the electrical current is 102 A.
solution, if the electrical current is 102 A.

