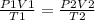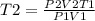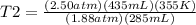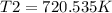Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
Syringe initially holds a sample of gas with a volume of 285 ml at 355 k and 1.88 atm. to what tempe...
Questions

Health, 19.09.2020 01:01




Social Studies, 19.09.2020 01:01

Social Studies, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

Computers and Technology, 19.09.2020 01:01


English, 19.09.2020 01:01

Biology, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

Chemistry, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01