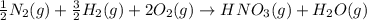Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) 2nh3(g) ah=-92. kj in the second step, ammonia and oxygen react to form nitric acid and water: nh3(9) + 2o2(g) → hno3(9) + h2o(g) ah=-330. kj calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest kj. пkj 1 x ś ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen...
Questions





Mathematics, 28.08.2019 09:10

Mathematics, 28.08.2019 09:10

Physics, 28.08.2019 09:10

Mathematics, 28.08.2019 09:10

Geography, 28.08.2019 09:10

Mathematics, 28.08.2019 09:10



History, 28.08.2019 09:10



Advanced Placement (AP), 28.08.2019 09:10

English, 28.08.2019 09:10



Mathematics, 28.08.2019 09:10

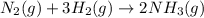 ,
, 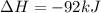
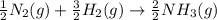 ,
, 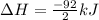
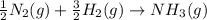 ,
, 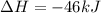 ............ (1)
............ (1)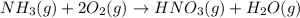
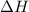 = -330 kJ ............ (2)
= -330 kJ ............ (2)