
Chemistry, 18.11.2019 20:31 Thunderalesis7855
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solution. first the student prepares a set of cocl2(aq) solutions of known concentration. then the student uses a spectrophotometer to determine the absorbance of each of the standard solutions at a wavelength of 510nm and constructs a standard curve. finally, the student determines the absorbance of the sample of unknown concentration. a wavelength of 510nm corresponds to an approximate frequency of 6×1014s−1. what is the approximate energy of one photon of this light? 9×1047j.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solu...
Questions


English, 29.04.2021 20:10

Mathematics, 29.04.2021 20:10

Biology, 29.04.2021 20:10



Mathematics, 29.04.2021 20:10

Mathematics, 29.04.2021 20:10

History, 29.04.2021 20:10

Mathematics, 29.04.2021 20:10

Mathematics, 29.04.2021 20:10


Mathematics, 29.04.2021 20:10




Mathematics, 29.04.2021 20:10

Mathematics, 29.04.2021 20:10

Computers and Technology, 29.04.2021 20:10

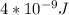
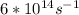
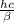 ---- ( 1 )
---- ( 1 )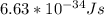
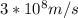
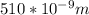
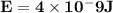
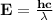
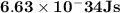
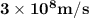
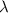 - wavelength = 510nm
- wavelength = 510nm


