
Chemistry, 18.11.2019 19:31 mixedgirlmara
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6 kj / mol rxn. using the following standard enthalpy of formation data, calculate standard enthalpy of formation for sic (s). a. standard enthalpy of formation sio2 (s) = -910.9 kj/mol b. standard enthalpy of formation co (g) = -110.5 kj/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
Consider the following equation: sio2 (s) + 3c (graphite) --> sic (s) + 2co (g) δh rxn = 624.6...
Questions


History, 06.09.2021 01:00

English, 06.09.2021 01:00

History, 06.09.2021 01:00

Mathematics, 06.09.2021 01:00

Chemistry, 06.09.2021 01:00


Social Studies, 06.09.2021 01:00




Health, 06.09.2021 01:00




Social Studies, 06.09.2021 01:00



Mathematics, 06.09.2021 01:00

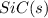 is coming out to be -65.3 kJ/mol
is coming out to be -65.3 kJ/mol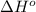
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0379/7554/72c39.png)
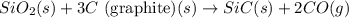
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times \Delta H^o_f_{(CO(g))})]-[(1\times \Delta H^o_f_{(SiO_2(s))})+(3\times \Delta H^o_f_{(C(s))})]](/tpl/images/0379/7554/6a7fe.png)
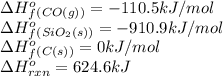
![624.6=[(1\times \Delta H^o_f_{(SiC(s))})+(2\times (-110.5))]-[(1\times (-910.9))+(3\times (0))]\\\\\Delta H^o_f_{(SiC(s))}=-65.3kJ/mol](/tpl/images/0379/7554/c8e18.png)


