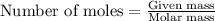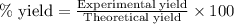Chemistry, 18.11.2019 19:31 puchie1225
A15.0 ml sample of a 1.92 m potassium sulfate solution is mixed with 14.9 ml of a 0.860 m barium nitrate solution and this precipitation reaction occurs: k2so4(aq) ba(no3)2(aq)→baso4(s) 2kno3(aq) the solid baso4 is collected, dried, and found to have a mass of 2.46 g . determine the limiting reactant, the theoretical yield, and the percent yield. part a determine the limiting reactant. express your answer as a chemical formula.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
A15.0 ml sample of a 1.92 m potassium sulfate solution is mixed with 14.9 ml of a 0.860 m barium nit...
Questions

Chemistry, 14.11.2019 10:31

Computers and Technology, 14.11.2019 10:31

Mathematics, 14.11.2019 10:31

Mathematics, 14.11.2019 10:31





Mathematics, 14.11.2019 10:31




Mathematics, 14.11.2019 10:31

Mathematics, 14.11.2019 10:31

Biology, 14.11.2019 10:31

Mathematics, 14.11.2019 10:31

History, 14.11.2019 10:31

Mathematics, 14.11.2019 10:31

Chemistry, 14.11.2019 10:31

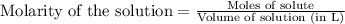 .....(1)
.....(1)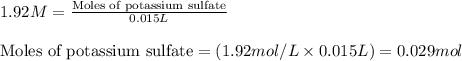

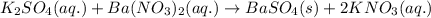
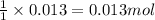 of potassium sulfate.
of potassium sulfate.