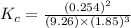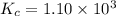Amixture of hydrogen and nitrogen, which produces ammonia (nh3) in a reaction vessel, is allowed to reach equilibrium at a given temperature. the equilibrium mixture of gases contained 0.254 m nh3, 1.85 m n2, and 9.26 m h2. calculate the equilibrium constant, kc at this temperature.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

You know the right answer?
Amixture of hydrogen and nitrogen, which produces ammonia (nh3) in a reaction vessel, is allowed to...
Questions

History, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50


Mathematics, 28.04.2021 16:50

English, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50


Mathematics, 28.04.2021 16:50



Mathematics, 28.04.2021 16:50


Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Biology, 28.04.2021 16:50

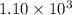
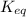
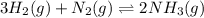
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0379/6771/c3aa0.png)