
Achemist mixes 1.00 g cucl2 with an excess of (nh4)2hpo4 in dilute aqueous solution . he measures the evolution of 670 j of heat as the two substances react to give cu3(po4)2(s). compute the δh that would result from the reaction of 1.00 mo! cucl2 with an excess of (nh4)2hpo4.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

You know the right answer?
Achemist mixes 1.00 g cucl2 with an excess of (nh4)2hpo4 in dilute aqueous solution . he measures th...
Questions


Mathematics, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01

Physics, 12.10.2020 23:01

Chemistry, 12.10.2020 23:01

Chemistry, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01








Mathematics, 12.10.2020 23:01




Mathematics, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01

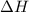 will be 90054 J
will be 90054 J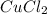 = 134.45 g/mol
= 134.45 g/mol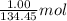 of
of 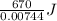 of heat or 90054 J of heat
of heat or 90054 J of heat

