Chemistry, 16.11.2019 19:31 anthonyfr10004
Asolution is prepared by mixed by mixing 0.10l of 0.14m sodium chloride with 0.21l of a 0.19m mgcl2 solution. what volume of 0.21m silver nitrate is required to precipitate all the cl- ion in the solution agcl?


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Asolution is prepared by mixed by mixing 0.10l of 0.14m sodium chloride with 0.21l of a 0.19m mgcl2...
Questions

Mathematics, 02.10.2020 20:01






Mathematics, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01

Computers and Technology, 02.10.2020 20:01

History, 02.10.2020 20:01

Chemistry, 02.10.2020 20:01




SAT, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01



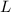
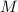 is the molarity and
is the molarity and 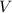 is the molarity of a given solution,
is the molarity of a given solution,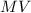 .
.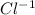 ions in the initial solution is the sum of number of moles of
ions in the initial solution is the sum of number of moles of 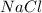 and
and 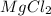


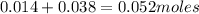
 of
of  ions are required to displace
ions are required to displace  ions.
ions.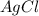 required is
required is