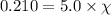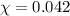Chemistry, 16.11.2019 07:31 Tyasiapalair371
Assume a scuba diver is working at a depth where the total pressure is 5.0 atm (about 50 m below the surface). what mole fraction of oxygen is necessary in order for the partial pressure of oxygen in the gas mixture the diver breathes to be 0.210 atm?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Assume a scuba diver is working at a depth where the total pressure is 5.0 atm (about 50 m below the...
Questions


Mathematics, 15.12.2020 19:40


Mathematics, 15.12.2020 19:40

Mathematics, 15.12.2020 19:40


Mathematics, 15.12.2020 19:40


Mathematics, 15.12.2020 19:40


English, 15.12.2020 19:40

Mathematics, 15.12.2020 19:40


Advanced Placement (AP), 15.12.2020 19:40

Mathematics, 15.12.2020 19:40

Arts, 15.12.2020 19:40



Law, 15.12.2020 19:40

Mathematics, 15.12.2020 19:40

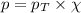
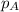 = partial pressure of oxygen = 0.210 atm
= partial pressure of oxygen = 0.210 atm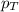 = total pressure = 5.0 atm
= total pressure = 5.0 atm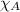 = mole fraction of oxygen = ?
= mole fraction of oxygen = ?