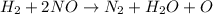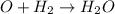Chemistry, 16.11.2019 06:31 arodriguez395
2h2(g) + 2no(g) → n2(g) + 2h2o(g) is rate=k[h2][no]2 (at least at low concentrations of h2). a possible mechanism for this reaction with two steps and an oxygen atom intermediate might take the form: h2 + 2no → n2 + x + o (step 1; k1) o + y → z (step 2; k2)
a. identify the chemical species x, y, and z in the mechanism.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
2h2(g) + 2no(g) → n2(g) + 2h2o(g) is rate=k[h2][no]2 (at least at low concentrations of h2). a possi...
Questions



Mathematics, 13.04.2021 03:30


Biology, 13.04.2021 03:30





Mathematics, 13.04.2021 03:30




Mathematics, 13.04.2021 03:30




Biology, 13.04.2021 03:30


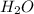 ,
, 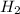 respectively.
respectively.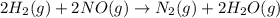
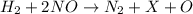
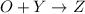
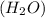 on right side of the reaction. So, the reaction 1 will be:
on right side of the reaction. So, the reaction 1 will be: