
Chemistry, 16.11.2019 05:31 isabelle1670
The neutralization of h3po4 with koh is exothermic. h3po4(aq)+3koh(aq)⟶3h2o(l)+k3po4(aq )+173.2 kj if 60.0 ml of 0.200 m h3po4 is mixed with 60.0 ml of 0.600 m koh initially at 23.43 °c, predict the final temperature of the solution, assuming its density is 1.13 g/ml and its specific heat is 3.78 j/(g·°c). assume that the total volume is the sum of the individual volumes.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
The neutralization of h3po4 with koh is exothermic. h3po4(aq)+3koh(aq)⟶3h2o(l)+k3po4(aq )+173.2 kj i...
Questions





History, 19.07.2019 14:00





English, 19.07.2019 14:00




Mathematics, 19.07.2019 14:00


History, 19.07.2019 14:00




English, 19.07.2019 14:00

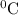
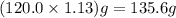
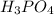 =
= 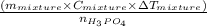
 represents change in temperature and n is number of moles
represents change in temperature and n is number of moles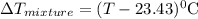
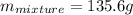 and
and 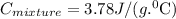
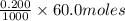 = 0.012 moles
= 0.012 moles![(173.2\times 10^{3})J=\frac{[(135.6g)\times (3.78J.g^{-1}.^{0}\textrm{C}^{-1})\times (T-23.43)^{0}\textrm{C}]}{0.012}](/tpl/images/0377/0998/64167.png)


