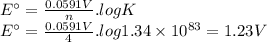The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 k is given below. 2 h2(g) + o2(g) → 2 h2o(l) k = 1.34 ✕ 1083 (a) calculate ℰ° and δg° at 298 k for the fuel-cell reaction. ℰ° v δg° kj (b) predict the signs of δh° and δs° for the fuel-cell reaction. δh°: positive negative δs°: positive negative (c) as temperature increases, does the maximum amount of work obtained from the fuel-cell reaction increase, decrease, or remain the same? explain. since δs is , as t increases, δg becomes more . therefore, the maximum work obtained will as t increases.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 14:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 6.00 mol fe and 8.45 mol nio(oh) react?
Answers: 1
You know the right answer?
The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 k is give...
Questions




Mathematics, 14.04.2020 16:07

Chemistry, 14.04.2020 16:07










Mathematics, 14.04.2020 16:07




History, 14.04.2020 16:07

Mathematics, 14.04.2020 16:07