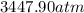Chemistry, 16.11.2019 03:31 prettyluhangel
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by the van der waals equation? the ideal gas law constant is 0.08206 [l•atm] / [mol•k]. for co2, the pressure correction constant is 3.658 l2•atm / mol 2, and the volume correction constant is 0.04286 l / mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by th...
Questions


English, 18.12.2020 19:50


Physics, 18.12.2020 19:50




Mathematics, 18.12.2020 19:50


Mathematics, 18.12.2020 19:50


English, 18.12.2020 19:50


Mathematics, 18.12.2020 19:50






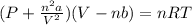
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}][1.0L-(20.0mol\times0.04286 L.mol^{-1} )]=(20.0mol)\times (0.08206L.atm.mol^{-1}.K^{-1})\times (300.0K)](/tpl/images/0376/9858/97073.png)
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}]](/tpl/images/0376/9858/353d5.png) =
=