

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2


Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3
You know the right answer?
A1.50 liter flask at a temperature of 25°c contains a mixture of 0.158 moles of methane, 0.09 moles...
Questions

Geography, 03.01.2021 14:00

Mathematics, 03.01.2021 14:00

Medicine, 03.01.2021 14:00



Chemistry, 03.01.2021 14:00

English, 03.01.2021 14:00

Mathematics, 03.01.2021 14:00

Chemistry, 03.01.2021 14:00


Biology, 03.01.2021 14:00



Mathematics, 03.01.2021 14:00


Physics, 03.01.2021 14:00

Geography, 03.01.2021 14:00

Mathematics, 03.01.2021 14:00


Mathematics, 03.01.2021 14:00

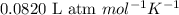
![25^oC=[25+273]K=298K](/tpl/images/0232/8594/df1f6.png)



