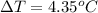High-purity benzoic acid (c6h5cooh; δhrxn for combustion = −3227 kj/mol) is used as a standard for calibrating bomb calorimeters. a 1.221-g sample burns in a calorimeter (heat capacity = 1365 j/°c) that contains exactly 1.430 kg of water. what temperature change is observed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
High-purity benzoic acid (c6h5cooh; δhrxn for combustion = −3227 kj/mol) is used as a standard for...
Questions

Computers and Technology, 30.11.2019 02:31
















Biology, 30.11.2019 02:31


English, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

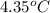

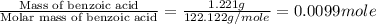
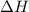 = enthalpy of combustion = 3227 kJ/mole
= enthalpy of combustion = 3227 kJ/mole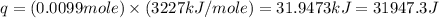
![q=[q_1+q_2]](/tpl/images/0376/8173/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0376/8173/1d50b.png)
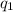 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter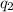 = heat absorbed by the water
= heat absorbed by the water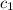 = specific heat of calorimeter =
= specific heat of calorimeter = 
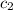 = specific heat of water =
= specific heat of water = 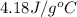
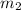 = mass of water = 1.430 kg = 1430 g
= mass of water = 1.430 kg = 1430 g = change in temperature = ?
= change in temperature = ?![31947.3J=[(1365J/^oC\times \Delta T)+(1430g\times 4.18J/g^oC\times \Delta T)]](/tpl/images/0376/8173/c2161.png)