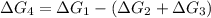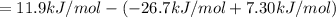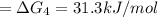Chemistry, 16.11.2019 00:31 dianasmygova
In nature, one common strategy to make thermodynamically unfavorable reactions proceed is to couple them chemically to reactions that are thermodynamically favorable. as long as the overall reaction is thermodynamically favorable, even the unfavorable reaction will proceed.
part a: consider these hypothetical chemical reactions:
a⇌b,δg= 11.9 kj/mol
b⇌c,δg= -26.7 kj/mol
c⇌d,δg= 7.30 kj/mol
what is the free energy, δg, for the overall reaction, a⇌d?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

You know the right answer?
In nature, one common strategy to make thermodynamically unfavorable reactions proceed is to couple...
Questions



Mathematics, 17.09.2019 05:00




Mathematics, 17.09.2019 05:00



History, 17.09.2019 05:00

Mathematics, 17.09.2019 05:00


Physics, 17.09.2019 05:00

Mathematics, 17.09.2019 05:00

Social Studies, 17.09.2019 05:00



Physics, 17.09.2019 05:00


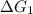 = 11.9 kJ/mol ...[1]
= 11.9 kJ/mol ...[1]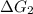 = -26.7 kJ/mol ...[2]
= -26.7 kJ/mol ...[2]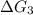 = 7.30 kJ/mol ...[3]
= 7.30 kJ/mol ...[3]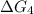 = ?...[4]
= ?...[4]