
Chemistry, 16.11.2019 00:31 samueltaye
In which one of the following processes is δh = δe? a. 2hi(g) => h2(g) + i2(g) at atmospheric pressure. b. two moles of ammonia gas are cooled from 325 ? °c to 300 °c at 1.2 atm. c. h2o(l) => h2o(g) at 100 °c at atmospheric pressure. d. caco3(s) => cao(s) + co2(g) at 800 °c at atmospheric pressure. e. co2(s) => co2(g) at atmospheric pressure.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

You know the right answer?
In which one of the following processes is δh = δe? a. 2hi(g) => h2(g) + i2(g) at atmospheric p...
Questions

History, 19.07.2020 23:01



Computers and Technology, 20.07.2020 01:01







Mathematics, 20.07.2020 01:01





English, 20.07.2020 01:01



Mathematics, 20.07.2020 01:01

Mathematics, 20.07.2020 01:01

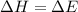
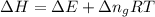
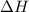 = change in enthalpy
= change in enthalpy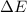 = change in internal energy
= change in internal energy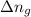 = change in moles
= change in moles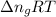 will be zero.
will be zero.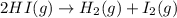 at atmospheric pressure.
at atmospheric pressure.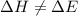
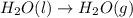 at 100 °C at atmospheric pressure.
at 100 °C at atmospheric pressure.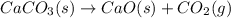 at 800 °C at atmospheric pressure.
at 800 °C at atmospheric pressure.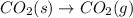 at atmospheric pressure.
at atmospheric pressure.

