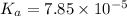Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

You know the right answer?
1.50 g of a weak acid (molar mass 176) is dissolved in 50.0 ml of water, and the resultant solution...
Questions

Mathematics, 30.11.2021 02:10

English, 30.11.2021 02:10


Mathematics, 30.11.2021 02:10

Mathematics, 30.11.2021 02:20


Computers and Technology, 30.11.2021 02:20

Mathematics, 30.11.2021 02:20


Social Studies, 30.11.2021 02:20



SAT, 30.11.2021 02:20



Mathematics, 30.11.2021 02:20

Mathematics, 30.11.2021 02:20

SAT, 30.11.2021 02:20


English, 30.11.2021 02:20

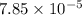 .
.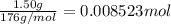
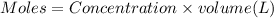
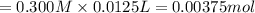
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0376/5102/e4eea.png)

![pH=pK_a+\log(\frac{[NaA]}{[HA]})](/tpl/images/0376/5102/83d67.png)
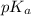 = ?
= ?![[HA]=\frac{0.008523 mol-0.00375}{0.0625 L}=\frac{0.004773 mol}{0.0625 L}](/tpl/images/0376/5102/6fe77.png)
![[NaA]=\frac{0.00375 mol}{0.0625 L}](/tpl/images/0376/5102/93c44.png)
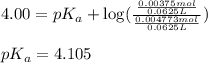
![4.105=-\log[K_a]](/tpl/images/0376/5102/b305f.png)