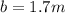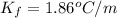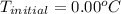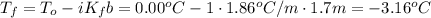Chemistry, 15.11.2019 20:31 elizabethhubbe
What would be the freezing point of a 1.7 mole aqueous ethylene glycol solution? the freezing point depression constant for water = 1.86 degrees celsius per mole.
a) 3.2°c
b) – 1.1°c
c) 0.0°c
d) – 3.2°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
What would be the freezing point of a 1.7 mole aqueous ethylene glycol solution? the freezing point...
Questions

Health, 05.04.2021 05:20



Arts, 05.04.2021 05:20

History, 05.04.2021 05:20


Mathematics, 05.04.2021 05:20

English, 05.04.2021 05:20


Mathematics, 05.04.2021 05:20



Mathematics, 05.04.2021 05:20

Chemistry, 05.04.2021 05:20

Physics, 05.04.2021 05:20

Business, 05.04.2021 05:20

English, 05.04.2021 05:20



History, 05.04.2021 05:20

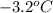

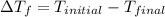 is the change in the freezing point of the solvent given its initial and final freezing point temperature values;
is the change in the freezing point of the solvent given its initial and final freezing point temperature values; is the van 't Hoff factor (i = 1 for non-electrolyte solutes and i depends on the number of moles of ions released per mole of ionic salt);
is the van 't Hoff factor (i = 1 for non-electrolyte solutes and i depends on the number of moles of ions released per mole of ionic salt);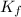 is the freezing point depression constant for the solvent;
is the freezing point depression constant for the solvent; is molality of the solute, defined as a ratio between the moles of solute and the mass of solvent (in kilograms).
is molality of the solute, defined as a ratio between the moles of solute and the mass of solvent (in kilograms).