Chemistry, 15.11.2019 20:31 Mrblunt5613
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g) δh o rxn = −1255.8 kj given δh o f of co2(g) = −393.5 kj/mol and δh o f of h2o(g) = −241.8 kj/mol, find δh o f of c2h2(g).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3


Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1
You know the right answer?
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g)...
Questions


History, 30.04.2021 14:00

Social Studies, 30.04.2021 14:00

Chemistry, 30.04.2021 14:00


Mathematics, 30.04.2021 14:00

Computers and Technology, 30.04.2021 14:00

Computers and Technology, 30.04.2021 14:00

Health, 30.04.2021 14:00

Biology, 30.04.2021 14:00


Business, 30.04.2021 14:00




Physics, 30.04.2021 14:00

English, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00


Geography, 30.04.2021 14:00

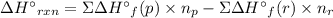
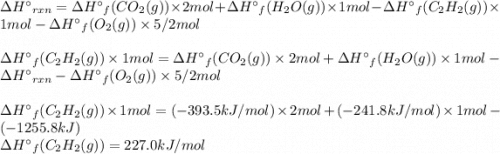
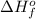 , of C₂H₂ is 227 kJ/mol
, of C₂H₂ is 227 kJ/mol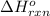 = −1255.8 kJ
= −1255.8 kJ