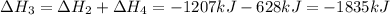Chemistry, 15.11.2019 19:31 makayyafreeman
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: p4(g) + 10 cl2(g) → 4pcl5(s) δh°rxn = ? given: pcl5(s) → pcl3(g) + cl2(g) δh°rxn= +157 kj p4(g) + 6 cl2(g) → 4 pcl3(g) δh°rxn = -1207 kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: p4...
Questions


Mathematics, 28.09.2020 21:01


Geography, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

Spanish, 28.09.2020 21:01



English, 28.09.2020 21:01

Chemistry, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01


Biology, 28.09.2020 21:01

English, 28.09.2020 21:01

Social Studies, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01


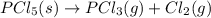
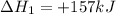 (1)
(1)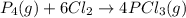
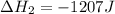 (2)
(2)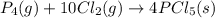
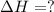 (3)
(3)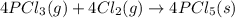
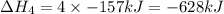 (4)
(4)