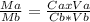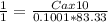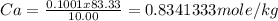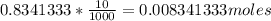Chemistry, 15.11.2019 19:31 jnsebastian2002
Vinegar is a dilute solution ethanoic acid in water (see the question above for the structure of ethanoic acid). in order to test the strength of a vinegar, a 10.00 gram sample was titrated with sodium hydroxide (0.1001 mole/kg of solution). the mass of the. burette before the titration is 131.44 g and upon reaching the endpoint the burette weighted 48.11 g. how many moles of acetic acid are in the sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Vinegar is a dilute solution ethanoic acid in water (see the question above for the structure of eth...
Questions

History, 31.01.2022 04:20

Mathematics, 31.01.2022 04:20


Mathematics, 31.01.2022 04:20




English, 31.01.2022 04:20

Mathematics, 31.01.2022 04:30



Spanish, 31.01.2022 04:30


Mathematics, 31.01.2022 04:30





Mathematics, 31.01.2022 04:30