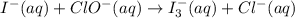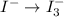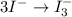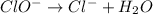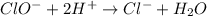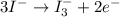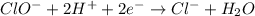Chemistry, 15.11.2019 19:31 truelove9288
Using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq) → i3-(aq) + cl-(aq) indicate the correct equation below. view available hint(s) using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq) → i3-(aq) + cl-(aq) indicate the correct equation below. 3i-(aq)+2h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+h2o(l) 3i-(aq)+2h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+h2o(l)+2e- i-(aq)+10h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+10h2o(l) i-(aq)+2h+(aq)+clo-(aq)→6i3-(aq)+cl -(aq)+h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1


Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq)...
Questions

Mathematics, 16.12.2020 21:20








World Languages, 16.12.2020 21:20

Biology, 16.12.2020 21:20

Advanced Placement (AP), 16.12.2020 21:20


Mathematics, 16.12.2020 21:20

Social Studies, 16.12.2020 21:20


Mathematics, 16.12.2020 21:20

English, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20

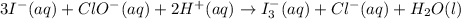
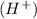 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.