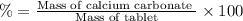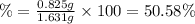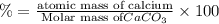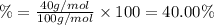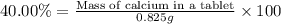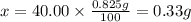Chemistry, 15.11.2019 07:31 harmonyfern5648
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. after the fillers were filtered out, the hcl was neutralized by adding sodium carbonate. the resulting precipitate was pure calcium carbnonate (with the fillers removed). the solid calcium carbonate was collected on a watch glass that had a mass of 46.719 g when empty. after teh calcium carbonate had been allowed to dry, the mass of the watch glass plus product was found to be 47.544 g.
1. what is the mass of pure calcium carbonate product collected at the end of the experiment?
2. calculate the mass % calium carbonate in the tablet.
3. calculate the mass percent calcium in calcium carbonate. this calculation is theoretical and is independent of the data provided.
4. calculate the number of grams of calcium that were in the tablet.
(hint: this can be obtained by using the answers to question 1 and 3)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

You know the right answer?
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. afte...
Questions

Mathematics, 16.01.2020 20:31


History, 16.01.2020 20:31




Geography, 16.01.2020 20:31


Biology, 16.01.2020 20:31

Arts, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31

History, 16.01.2020 20:31

Health, 16.01.2020 20:31



English, 16.01.2020 20:31