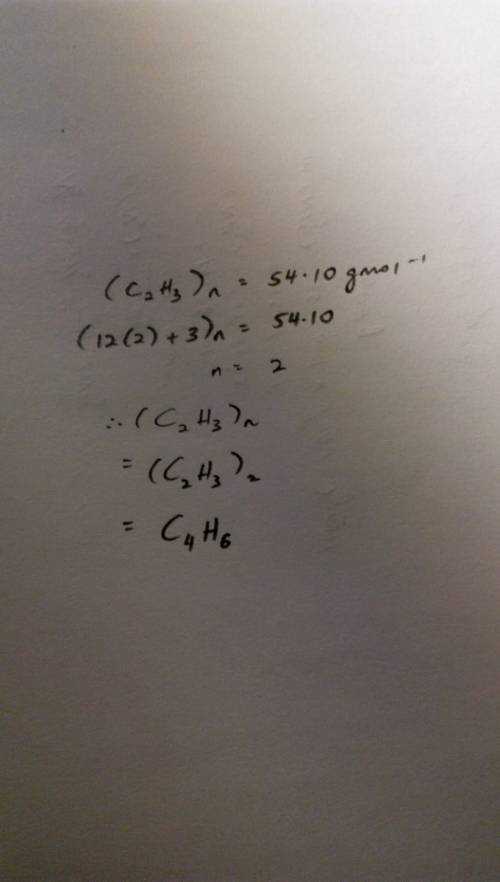Chemistry, 26.08.2019 12:30 kiarabermudez754
The empirical formula of a compound is determined to be c2h3, and its molecular mass is found to be 54.10 g/mol. determine the molecular formula of the compound, showing your work.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
The empirical formula of a compound is determined to be c2h3, and its molecular mass is found to be...
Questions

History, 12.01.2021 06:20

Mathematics, 12.01.2021 06:20

Chemistry, 12.01.2021 06:20





History, 12.01.2021 06:20

Mathematics, 12.01.2021 06:20

History, 12.01.2021 06:20

Mathematics, 12.01.2021 06:20



Mathematics, 12.01.2021 06:20

Mathematics, 12.01.2021 06:20

Chemistry, 12.01.2021 06:20




English, 12.01.2021 06:20