
Chemistry, 15.11.2019 04:31 hannahgracew12
Consider the equilibrium reaction below. at high concentrations of ha the solution will be yellow; but at low concentrations of ha the solution will be red. initially the equilibrium mixture below is orange (combination of yellow & red). what color is expected after adding some colorless a–(aq) to the equilibrium solution? a-(aq) + h2o(ℓ) ⇋ ha(aq) + oh-(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
Consider the equilibrium reaction below. at high concentrations of ha the solution will be yellow;...
Questions

Mathematics, 26.04.2021 22:30

Biology, 26.04.2021 22:30



Health, 26.04.2021 22:30




Social Studies, 26.04.2021 22:30




Mathematics, 26.04.2021 22:30



Mathematics, 26.04.2021 22:30




Mathematics, 26.04.2021 22:30

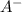 is added into the solution then it means that we are increasing the amount of reactant.
is added into the solution then it means that we are increasing the amount of reactant. 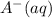 to the equilibrium solution.
to the equilibrium solution.

