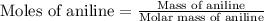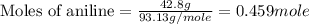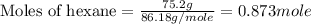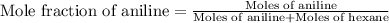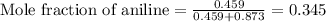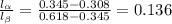Aniline and hexane form partially miscible liquid-liquid mixtures below 69 ºc. when 42.8 g of aniline and 75.2 g of hexane are mixed at 67.5 ºc, two separate liquid phases are formed, with mole fractions of aniline of 0.308 and 0.618. determine the overall mole fraction of aniline in the mixture, and then use the lever rule to determine the relative amounts of the two phases.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Aniline and hexane form partially miscible liquid-liquid mixtures below 69 ºc. when 42.8 g of anilin...
Questions

Arts, 05.11.2020 22:00

Chemistry, 05.11.2020 22:00




Geography, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00


English, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00

English, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00

Social Studies, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00