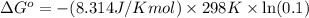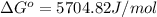Calculate δg°' for the reaction a + b < -> c + d at 25°c when the equilibrium concentrations are [a] = 10 um, [b] = 15 um, [c] = 3 um, and [d] = 5 um. r = 8.314 j/mol⋅k. give your answer to the nearest hundredths in j/mol, but only provide the numerical value (i. e. don't include units in your answer).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Calculate δg°' for the reaction a + b < -> c + d at 25°c when the equilibrium concentrations...
Questions

Mathematics, 15.10.2019 02:40

History, 15.10.2019 02:40



English, 15.10.2019 02:40



Biology, 15.10.2019 02:40

Social Studies, 15.10.2019 02:40




Mathematics, 15.10.2019 02:40

History, 15.10.2019 02:40

Social Studies, 15.10.2019 02:40


Biology, 15.10.2019 02:40


English, 15.10.2019 02:40

![K=\frac{[C][D]}{[A][B]}](/tpl/images/0375/2516/80561.png)
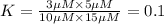
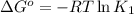
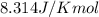
![25^oC=[273+25]K=298K](/tpl/images/0375/2516/0e82f.png)
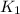 = equilibrium constant at 25°C = 0.1
= equilibrium constant at 25°C = 0.1