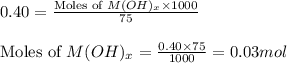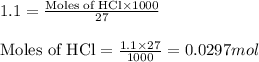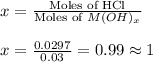The solid oxide of generic metal m is added to 75 ml of water and reacts to produce a metal hydroxide solution that is 0.40 m in the resulting compound. the metal hydroxide solution then reacts with all of the 27 ml of 1.1 m hcl to form water and the metal salt. how many valence electrons must m have?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
The solid oxide of generic metal m is added to 75 ml of water and reacts to produce a metal hydroxid...
Questions

Biology, 20.04.2021 02:00


Mathematics, 20.04.2021 02:00



Mathematics, 20.04.2021 02:00

Social Studies, 20.04.2021 02:00

Mathematics, 20.04.2021 02:00

Mathematics, 20.04.2021 02:00

English, 20.04.2021 02:00

Social Studies, 20.04.2021 02:00



Mathematics, 20.04.2021 02:00






Biology, 20.04.2021 02:00

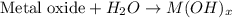
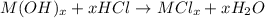
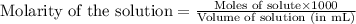 .....(1)
.....(1)