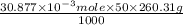Chemistry, 14.11.2019 20:31 SucMaDongShan
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (free acid) required to prepare 50 ml of a 100 mm buffer that is ph = 7.20. the pka of hepes is 7.55 at 20° c. the formula weight of the sodium salt is 260.31. the formula weight of the free acid is 238.31. weigh out the appropriate amounts of the hepes (sodium salt) and hepes (free acid), transfer to a 100 ml beaker, dissolve in deionized water to an approximate volume of 40 ml

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (fre...
Questions



Mathematics, 05.12.2020 04:20


Mathematics, 05.12.2020 04:20

Mathematics, 05.12.2020 04:20






Mathematics, 05.12.2020 04:20

Biology, 05.12.2020 04:20

Chemistry, 05.12.2020 04:20

Social Studies, 05.12.2020 04:20

Mathematics, 05.12.2020 04:20




Computers and Technology, 05.12.2020 04:20

![pK_{a} + log \frac{[Salt]}{[Acid]}](/tpl/images/0374/5521/81f72.png)
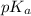 = 7.55.
= 7.55.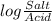
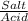 = 0.4467
= 0.4467 = 100 mM
= 100 mM 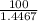
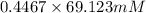
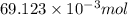 .
.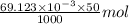
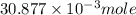 .
.