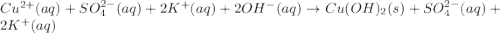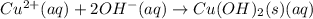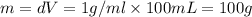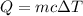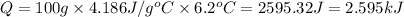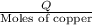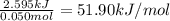Consider two solutions, the first being 50.0 ml of 1.00 m cuso4 and the second 50.0 ml of 2.00 m koh. when the two solutions are mixed in a constant-pressure calorimeter, a precipitate forms and the temperature of the mixture rises from 21.5 ∘c to 27.7 ∘c.
a) before mixing, how many grams of cu are present in the solution of cuso4?
b) predict the identity of the precipitate in the reaction.
c) write complete equation for the reaction that occurs when the two solutions are mixed.
d) write net ionic equation for the reaction that occurs when the two solutions are mixed.
e) from the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the calorimeter absorbs only a negligible quantity of heat, that the total volume of the solution is 100.0 ml, and that the specific heat and density of the solution after mixing are the same as that of pure water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

You know the right answer?
Consider two solutions, the first being 50.0 ml of 1.00 m cuso4 and the second 50.0 ml of 2.00 m koh...
Questions

Mathematics, 20.08.2019 22:30



Mathematics, 20.08.2019 22:30

Social Studies, 20.08.2019 22:30






Computers and Technology, 20.08.2019 22:30






Computers and Technology, 20.08.2019 22:30



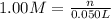
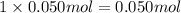 of copper
of copper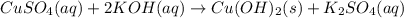
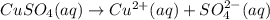 ..[1]
..[1]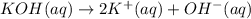 ..[2]
..[2]