
Chemistry, 23.08.2019 20:40 hbkakabryce0p3fkoq
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions

Mathematics, 20.11.2020 14:00

Computers and Technology, 20.11.2020 14:00


Biology, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00


Spanish, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00



Mathematics, 20.11.2020 14:00

Physics, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00


Mathematics, 20.11.2020 14:00

History, 20.11.2020 14:00

Social Studies, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00

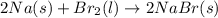
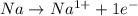
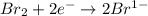
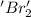 is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is, 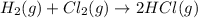
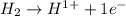

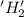 is oxidized and
is oxidized and 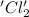 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 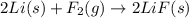
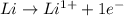
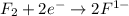
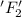 is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is, 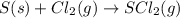
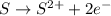
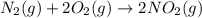
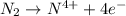

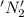 is oxidized and
is oxidized and 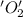 is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 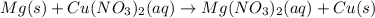
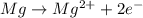
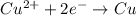
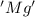 is oxidized and
is oxidized and 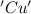 is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.
is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.

