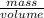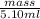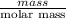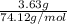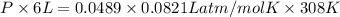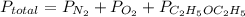Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l vessel that already contains a mixture of n2 and o2, whose partial pressures are pn2 = 0.752 atm and po2 = 0.206 atm. the temperature is held at 35.0 °c, and the diethylether totally evaporates.
a) calculate the partial pressure of the diethylether.
b) calculate the total pressure in the container.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l...
Questions

English, 09.12.2020 03:30

English, 09.12.2020 03:30




Mathematics, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30

History, 09.12.2020 03:30


Mathematics, 09.12.2020 03:30


English, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30



Chemistry, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30


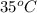 = (35 + 273) K = 308 K
= (35 + 273) K = 308 K