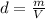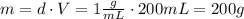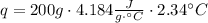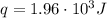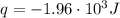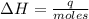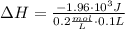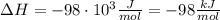Chemistry, 14.11.2019 06:31 lexybellx3
A1.00 x 102ml sample of 0.200 m aqueous hydrochloric acid is added to 1.00 x 102ml of 0.200 m aqueous ammonia in a constant-pressure calorimeter of negligible heat capacity. the following reaction occurs when the two solutionsare mixedhcl(aq)+ nh3(> nh4cl(aq)the temperature increase is 2.34°c. calculate heat change of the reaction per mole of hcl reacted. assume that the densities and specific heats of the solutions are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
A1.00 x 102ml sample of 0.200 m aqueous hydrochloric acid is added to 1.00 x 102ml of 0.200 m aqueou...
Questions




Biology, 15.07.2019 23:30

Chemistry, 15.07.2019 23:30

Social Studies, 15.07.2019 23:30


Advanced Placement (AP), 15.07.2019 23:30


Health, 15.07.2019 23:30



Computers and Technology, 15.07.2019 23:30



Social Studies, 15.07.2019 23:30

Biology, 15.07.2019 23:30



Computers and Technology, 15.07.2019 23:30

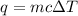 (1)
(1)