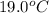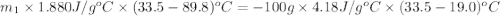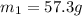Chemistry, 14.11.2019 05:31 saabrrinnaaa
Asample ofpolystyrene, which has a specific heat capacity of 1.880 j/g k, is put into a calorimeter that contains 100.0 g of water. the polystyrene sample starts off at 89.8 c and the temperature of water starts off at 19.0°c. when the temperature of the water stops changing it is 33.5°c. the pressure remains constant at 1.0 atm. calculate the mass of the polystyrene sample. be sure your answer is rounded to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
Asample ofpolystyrene, which has a specific heat capacity of 1.880 j/g k, is put into a calorimeter...
Questions





Social Studies, 23.09.2020 19:01


Mathematics, 23.09.2020 19:01


History, 23.09.2020 19:01

Social Studies, 23.09.2020 19:01

Mathematics, 23.09.2020 19:01



English, 23.09.2020 19:01



Mathematics, 23.09.2020 19:01

History, 23.09.2020 19:01


History, 23.09.2020 19:01

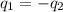
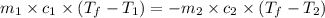
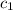 = specific heat of polystyrene =
= specific heat of polystyrene = 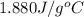
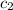 = specific heat of water =
= specific heat of water = 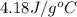
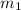 = mass of polystyrene = ?
= mass of polystyrene = ?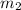 = mass of water = 100.0 g
= mass of water = 100.0 g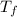 = final temperature of system =
= final temperature of system = 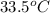
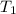 = initial temperature of polystyrene =
= initial temperature of polystyrene = 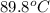
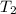 = initial temperature of water =
= initial temperature of water =