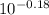Chemistry, 14.11.2019 03:31 THEOUTCOMER
Abuffer is prepared by adding 150 ml of 1.0 m naoh to 250 ml of 1.0 m nah2po4. how many moles of hcl must be added to this buffer solution to change the ph by 0.18 units? assume the total volume remains unchanged at 400 ml. for h2po4-, ka2 = 6.3 × 10^-8.
o 0.063 mol
o 1.0 mol
o 0.025 mol
o 0.082 mol
o 0.50 mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1


Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Abuffer is prepared by adding 150 ml of 1.0 m naoh to 250 ml of 1.0 m nah2po4. how many moles of hcl...
Questions

Mathematics, 03.09.2020 06:01




Geography, 03.09.2020 06:01


Mathematics, 03.09.2020 06:01



English, 03.09.2020 06:01

Mathematics, 03.09.2020 06:01

English, 03.09.2020 06:01

Social Studies, 03.09.2020 06:01


History, 03.09.2020 06:01


Mathematics, 03.09.2020 06:01

Business, 03.09.2020 06:01

Mathematics, 03.09.2020 06:01