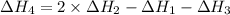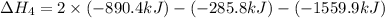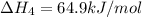1) the heat of combustion for the gases hydrogen, methane and ethane are −285.8, −890.4 and −1559.9 kj/mol respectively at 298k.
equation 1 h2 + 1⁄2o2 > h2o δh = −285.8 kj
equation 2 ch4 + 2o2 > co2 + 2h2o δh = −890.4 kj
equation 3 c2h6 + 7⁄2o2 > 2co2 + 3h2o δh = −1559.9 kj
use the above equations to calculate (at the same temperature) the heat of reaction for the following reaction:
2ch4(g) > c2h6(g) + h2(g)
solution:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


You know the right answer?
1) the heat of combustion for the gases hydrogen, methane and ethane are −285.8, −890.4 and −1559.9...
Questions


Chemistry, 10.07.2021 14:00


Physics, 10.07.2021 14:00


History, 10.07.2021 14:00


Mathematics, 10.07.2021 14:00



Chemistry, 10.07.2021 14:00



Geography, 10.07.2021 14:00






 ..[1]
..[1]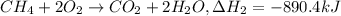 ..[2]
..[2]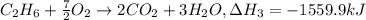 ..[3]
..[3]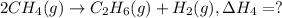 ..[4]
..[4]