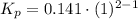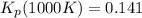Chemistry, 13.11.2019 06:31 SisterMina
Consider the equilibrium c2h6(g) ↔ c2h4(g) + h2(g) . at 1000k and a constant total pressure of 1 bar, h2(g) is introduced into the reaction vessel. the total pressure is held constant at 1 bar and at equilibrium the composition of the mixture in mole percent is h2 : 26% ; c2h4: 26% ; c2h6 : 48%calclate kp at 1000 k.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1


Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Consider the equilibrium c2h6(g) ↔ c2h4(g) + h2(g) . at 1000k and a constant total pressure of 1 bar...
Questions

Chemistry, 20.01.2021 04:10

Spanish, 20.01.2021 04:10



Mathematics, 20.01.2021 04:10



Computers and Technology, 20.01.2021 04:10

Mathematics, 20.01.2021 04:10


Health, 20.01.2021 04:10

Mathematics, 20.01.2021 04:10

Mathematics, 20.01.2021 04:10



Biology, 20.01.2021 04:10

Biology, 20.01.2021 04:10


Mathematics, 20.01.2021 04:10

Mathematics, 20.01.2021 04:10

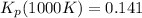
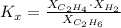
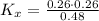
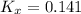
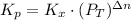
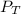 : total pressure and Δn: number of gaseous moles of product - number of gaseous moles of reactant
: total pressure and Δn: number of gaseous moles of product - number of gaseous moles of reactant