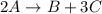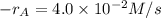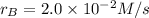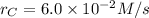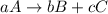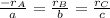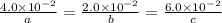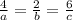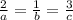Chemistry, 13.11.2019 05:31 ninilizovtskt
At a certain time in a reaction, substance a is disappearing at a rate of 4.0×10−2 m/s, substance b is appearing at a rate of 2.0×10−2 m/s, and substance c is appearing at a rate of 6.0×10−2 m/s. which of the following could be the stoichiometry for the reaction being studied?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2
You know the right answer?
At a certain time in a reaction, substance a is disappearing at a rate of 4.0×10−2 m/s, substance b...
Questions

Mathematics, 18.04.2021 08:00


Biology, 18.04.2021 08:00

Mathematics, 18.04.2021 08:00





Physics, 18.04.2021 08:00




Mathematics, 18.04.2021 08:00


English, 18.04.2021 08:00



Mathematics, 18.04.2021 08:00

English, 18.04.2021 08:00

Mathematics, 18.04.2021 08:00