
Chemistry, 13.11.2019 05:31 SushiMagic
Propane is burned completely with excess oxygen. the product gas contains 24.8 mole% co2, 6.12% co, 40.8% h2o, and 28.6% o2.
a) calculate the percentage excess o2 fed to the furnace.
b) a student wrote the stiochemtric equation of the combustion of propane to form co2 and co as
2c3h8 + 17/2o2 > 3co2 + 3co +8h20
according to this equation, co2 and co should be a ratio of 1/1 in the reaction products, but in the product gas of part (a) they are in a ratio of 24.8/6.12. is the result possible? (hint: yes) explain how.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
Propane is burned completely with excess oxygen. the product gas contains 24.8 mole% co2, 6.12% co,...
Questions

Mathematics, 25.05.2021 04:00


Business, 25.05.2021 04:00






History, 25.05.2021 04:00

Chemistry, 25.05.2021 04:00

Mathematics, 25.05.2021 04:00


Mathematics, 25.05.2021 04:00

Mathematics, 25.05.2021 04:00


Mathematics, 25.05.2021 04:00

Mathematics, 25.05.2021 04:00

Mathematics, 25.05.2021 04:00

Mathematics, 25.05.2021 04:00

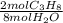 = 10,2 molesC₃H₈
= 10,2 molesC₃H₈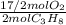 = 43,35 mol O₂
= 43,35 mol O₂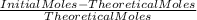 ×100
×100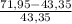 ×100 = 66% excess of O₂
×100 = 66% excess of O₂

