
Chemistry, 13.11.2019 04:31 friendsalwaysbae
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g) hexane is a low-boiling point component of gasoline. answer the question in part 1 through part 3, if the rate of reaction of c6h14 is 1.95 mol l-1s-1. the rate of reaction of c6h14 is the rate of disappearance of c6h14. what was the rate of formation of co2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
The balanced chemical equation for the combustion of hexane is: 2c6h14(l) 19o2(g) 12co2(g) 14h2o(g)...
Questions



Mathematics, 29.10.2020 22:30




Mathematics, 29.10.2020 22:30

Mathematics, 29.10.2020 22:30


History, 29.10.2020 22:30

Geography, 29.10.2020 22:30



Mathematics, 29.10.2020 22:30

Computers and Technology, 29.10.2020 22:30




History, 29.10.2020 22:30

English, 29.10.2020 22:30

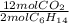 = 11,7 M/s
= 11,7 M/s

